How atoms hold hands and form the world around us.
Atoms aren’t meant to be alone. Just like people, they like to form relationships, and how they connect changes everything. From the water you drink to the steel in your phone, chemical bonds determine structure, function, and stability.
Today, we’ll explore how atoms bond, the three main types of bonds, what makes molecules polar or nonpolar, and how to draw atoms in a way that makes sense.
Why Do Atoms Bond?
Atoms bond to achieve stability, usually by filling their outermost energy shell (the valence shell) with electrons.
Think of it like this: every atom is playing a game of “Get to 8” (8 valence electrons = stable), and they’ll either give, take, or share electrons to do it.
This desire for stability leads to three types of bonds:
The 3 Types of Chemical Bonds
1. Ionic Bonding – The giver and the taker
One atom gives up one or more electrons, and another atom takes them. The result? Ions form and are attracted to each other due to their opposite charges.
- Usually between metals (givers) and nonmetals (takers)
- Creates charged atoms (ions):
- Cations = positive (lost electron)
- Anions = negative (gained electron)
- Forms crystalline solids like salt (NaCl)
Example: Sodium (Na) gives one electron to chlorine (Cl) → Na⁺ and Cl⁻ form NaCl.
2. Covalent Bonding– Sharing is caring
Here, atoms share electrons to fill their outer shells.
- Usually between nonmetals
- Forms molecules
- Can share 1, 2, or 3 pairs of electrons → single, double, triple bonds
Example: H₂O (water). Each hydrogen shares 1 electron with oxygen.
3. Metallic Bonding – The electron sea
In metals, atoms don’t just pair off. Instead, they release their valence electrons into a “sea” of electrons that flows freely between metal ions.
- Only in metals
- Explains why metals are:
- Conductive (electrons move easily)
- Malleable (bend without breaking)
- Shiny (reflective surfaces)
Example: Copper wires, gold rings, aluminum foil
Polar vs. Nonpolar Covalent Bonds
Even in covalent bonds, sharing isn’t always equal. Some atoms pull harder on electrons than others.
- Electronegativity is the atom’s “pulling power” for electrons.
Nonpolar Covalent Bonds:
- Electrons are shared equally.
- Happens between identical atoms or atoms with very similar electronegativity.
- Ex: O₂, N₂, CH₄
Polar Covalent Bonds:
- Electrons are shared unequally.
- One side becomes slightly negative (δ⁻), the other slightly positive (δ⁺).
- Ex: H₂O (oxygen hogs the electrons)
Polar molecules can form hydrogen bonds, which are responsible for water’s amazing properties (like surface tension, ice floating, and temperature regulation).
Lewis Dot Structures
Lewis structures are like diagrams of atoms showing only their valence electrons.
- Dots = valence electrons
- Shared pairs (bonds) = lines
- Lone pairs = dots that aren’t shared
Example: For water (H₂O), oxygen has 6 valence electrons and shares 2 with two hydrogen atoms.
The Lewis structure looks like this:
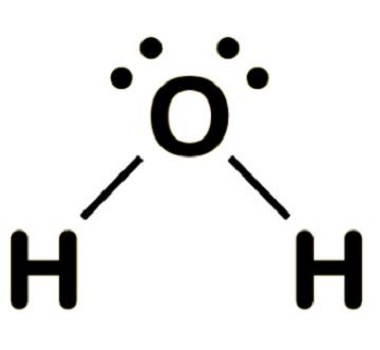
(Lone pairs sit on the oxygen like unshared roommates.)
Molecular Geometry (Basic Intro)
Bonding isn’t flat: molecules have 3D shapes that affect how they interact.
A quick preview of shapes:
- Linear: 2 atoms or 2 pairs around center (Ex: CO₂)
- Bent: 2 bonded + 2 lone pairs (Ex: H₂O)
- Trigonal planar: 3 bonds (Ex: BF₃)
- Tetrahedral: 4 bonds (Ex: CH₄)
The shape depends on electron repulsion, electrons push each other away, forming specific angles.
Recap Table:
| Bond Type | Involves | Found In | Example |
| Ionic | Electron transfer | Metal + Nonmetal | NaCl |
| Covalent | Electron sharing | Nonmetal + Nonmetal | H₂O, CO₂ |
| Metallic | Electron “sea” | Metal + Metal | Fe, Cu, Au |
| Polar Covalent | Unequal sharing | Slight charges formed | H₂O |
| Nonpolar | Equal sharing | No charge difference | O₂, CH₄ |
